



Table of Contents: 2024 MAY–JUNE No. 458
Study Record Information Displays in the Modernized ClinicalTrials.gov. NLM Tech Bull. 2024 May-Jun;(458):e7.
ClinicalTrials.gov study records consist of required and optional data elements that summarize a study's protocol and results information. Sponsors and investigators submit the information for their studies during registration and results reporting, respectively. Over the past 25 years, the data elements and their requirements have changed in response to the evolution of trial reporting laws and policies. Consequently, older ClinicalTrials.gov records may be missing information that was not being collected or was optional during initial submission but was subsequently added or became required. As we modernized the ClinicalTrials.gov website, we considered the display of information and the related explanations that would be most helpful to users, including how to display the data submitted by study sponsors and investigators and how to indicate the information they did not provide.
Each ClinicalTrials.gov record has a set of tabs that group study information (Figure 1).

Two tabs present information about study registration information. The Study Details tab displays an abstract of the study protocol, with sections for key information, such as Study Overview, Contacts and Locations, Participation Criteria, and Publications that can be accessed quickly using the navigation menu (displayed on left side of record). Generally, only information provided by the sponsor or investigator during study registration is displayed in this tab.
In contrast, the Researcher View tab provides tabular displays of all the protocol registration data elements (one per table row), even if no information was provided by the sponsor or investigator. This standardized format facilitates the rapid identification and analysis of specific data elements across study records. Because all currently available data elements are displayed on this tab, including optional ones and those that were optional at the time of registration but are now required, some rows include a note that information was not provided (Figure 2).
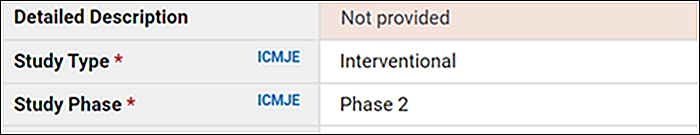
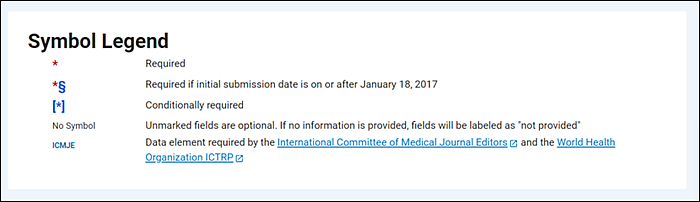
Researcher View also indicates the current status of each registration data element (required, conditionally required, required after the effective date of the Final Rule, or optional) with the same symbols used in the protocol registration data element definitions document. In addition, the Researcher View includes footnotes explaining the symbols (Figure 3).
The Results Posted tab, only available for studies with summary results, provides a tabular view of all results information that have been submitted for the study. If no results have been posted, a "Results Not Posted" tab takes the place of the "Results Posted" tab.
The Study Details and Researcher View tabs communicate information differently because they address distinct user needs. For example, the Researcher View tab displays and annotates all current ClinicalTrials.gov protocol registration data elements, whether or not information was provided by the sponsor or investigator.
On the Study Details tab, however, if information is not available for a study, the corresponding section will not appear in either the navigation menu or the body of the record. For example, the Publications section will not appear in the Study Details navigation menu for that study record when no publications have been listed or linked to PubMed (Figure 4).

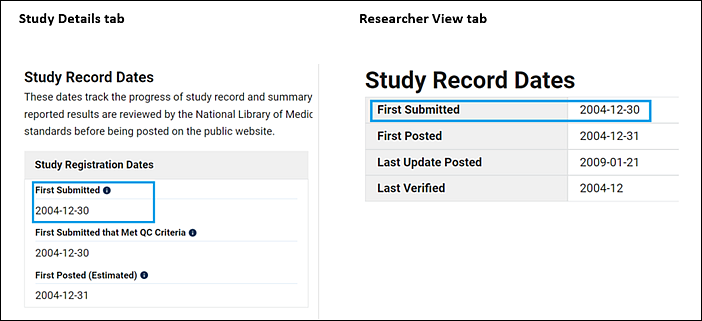
While the modernized ClinicalTrials.gov displays the information that has been submitted by the sponsor or investigator, the Researcher View also provides a complete listing of registration data elements available today with their current status (required or optional). The increased transparency offered by the displays of the modernized website reveals that the data elements and their requirements have changed considerably over the past 25 years. In the Researcher View tab, study records include the "not provided" note in fields that are currently required but lack information. However, it is possible that the data element itself did not exist or was optional at the time of protocol registration. To confirm when the study information was initially registered, check the First Submitted date in the Study Record Dates section on either the Study Details or Researcher View tab (Figure 5). In general, the older the First Submitted date, the more likely that the data elements and their requirements at the time of submission differ from those today.
If you haven't already, try out the modernized ClinicalTrials.gov and let us know what you think. We also encourage you to submit comments and suggestions using the yellow feedback button at the bottom right corner of the site's pages.