3: Exchange Standards
Clinical Document Architecture (CDA)

A clinical document is any document providing information about a patient or a group of patients, such as diagnostic findings from an interaction, a discharge summary, or a quality measure report regarding a group of patients.
The need to send and receive clinical documents in a structured format led HL7 to the creation of the Clinical Document Architecture (CDA).
XML-Based Format
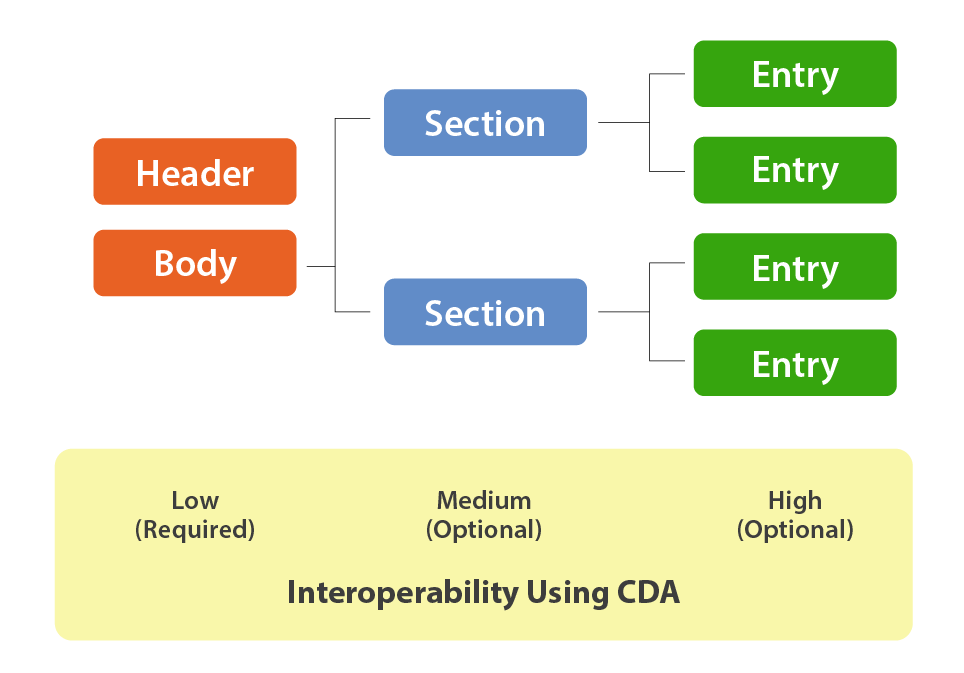
CDA is a document markup standard using XML that specifies the structure of clinical documents. The structure "wraps" information about the document and the document itself inside plain text XML tags. The structure includes:
Flexible Structure
The simplest CDA structure contains only the Header and Body, and those two components provide the lowest level of interoperability. For example, the Body could contain an Adobe PDF or a Microsoft Word document with the rendering and processing of which is left up to the receiving party. The Header provides structured descriptive information about the document contained in the Body so that the receiver can identify and use it.
As more structure is added into the Body with standardized nested body sections, more functionality and interoperability can be achieved. For example, a nursing SOAP (Subjective, Objective, Assessment and Plan) Note could be embedded in the body and include the recognizable structural sections or “narrative blocks” such as:
These clearly demarcated sections make the information in the document findable, displayable, and usable by humans and machines. Because of the XML format, the receiver needs only a web browser and what’s called a "style sheet" that has instructions for rendering the different sections of the CDA XML file.
At the highest level of interoperability, the CDA specification allows for the definition of subsections within the sections, called "Entries."
Semantic Interoperability
Entries can be highly specified, using the V3 Reference Information Model (RIM) to define information categories (entity, role, act, or participation). Entries can also use codes such as the terminologies introduced in the previous module. Use of standard terminologies such as LOINC or SNOMED CT within the document enhances semantic interoperability. Not only can documents be exchanged between different systems, but the content of those documents can be understood and used by these different systems. For example, an observation section may include a value describing a diagnosis defined by SNOMED-CT.
For example:
Note that, at this highest level of interoperability, the coding system and the specific concept are represented both by numeric identifiers (for machine translation) and by words (for human readability).
In fact, the narrative block is required by the CDA specification to maintain human readability of a document. This flexible support of incremental interoperability makes the CDA standard easy to implement at the level an institution is ready to use.
Consolidated Clinical Document Architecture (C-CDA)
Healthcare institutions need a standard CDA implementation guide for patient summaries, to communicate with other healthcare providers and to communicate with the patient via patient portals. They also need implementation guides for other communications such as discharge summaries and operative reports. HL7, working with ASTM International and others, created a Consolidated CDA (C-CDA) implementation guide to address these needs.
The C-CDA consolidated multiple document types (including an earlier implementation called the Continuity of Care Document) into a unified standard. The Promoting Interoperability Program standard uses C-CDA as a default export format for patient communication and coordination of care, bolstering its adoption.
Learn more about the CDA standard from the HL7 website.